Germán Newall, MD
4400 Post Oak Pkwy Suite 300
Houston, TX 77027
Phone: (713) 936-6971
M-F: 8:30am – 5:00pm
Capsular Contracture Study with Dr. Germán Newall – Now Enrolling
Dr. Germán Newall is proud to be a participating surgeon in this clinical study. This important research is evaluating a new treatment option for patients experiencing capsular contracture after breast augmentation. If you’ve experienced complications from implants and may qualify, this is an opportunity to receive advanced care with the support of cutting-edge technology.
Request a ConsultationWhat Is Capsular Contracture?
Capsular contracture is the most common reason for reoperation after breast implant surgery. It occurs when the scar tissue that naturally forms around a breast implant tightens, causing the breast to feel firm, look misshapen, or become painful. Capsular contracture is graded from mild (Grade I/II) to severe (Grade III/IV). Patients with Grade III and IV often require surgical revision.
Request a Consultation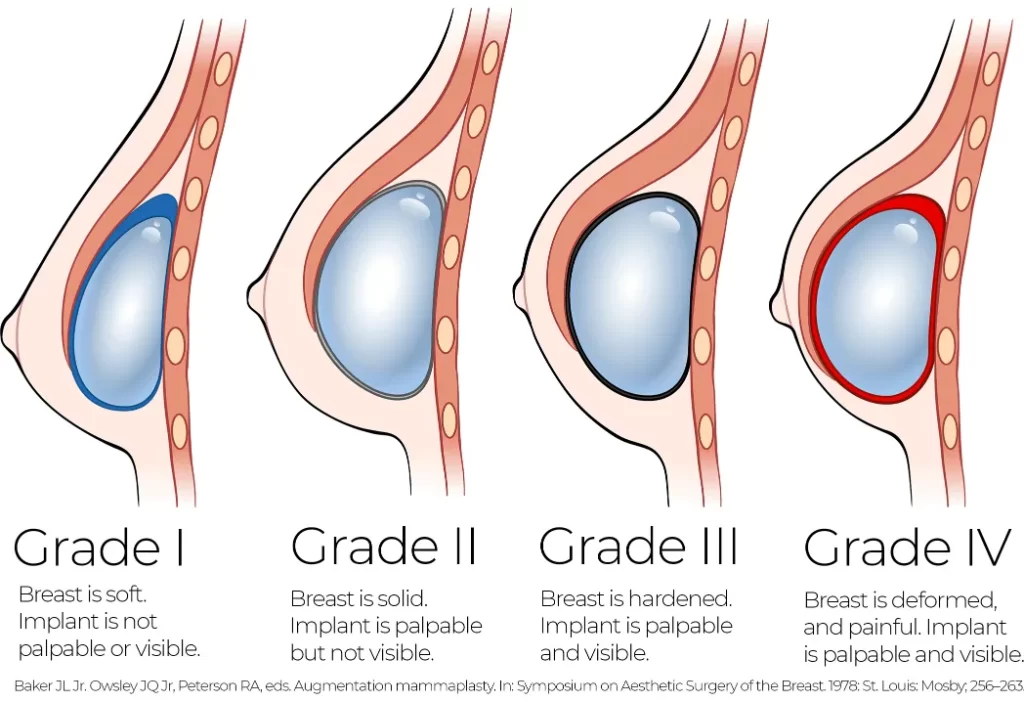
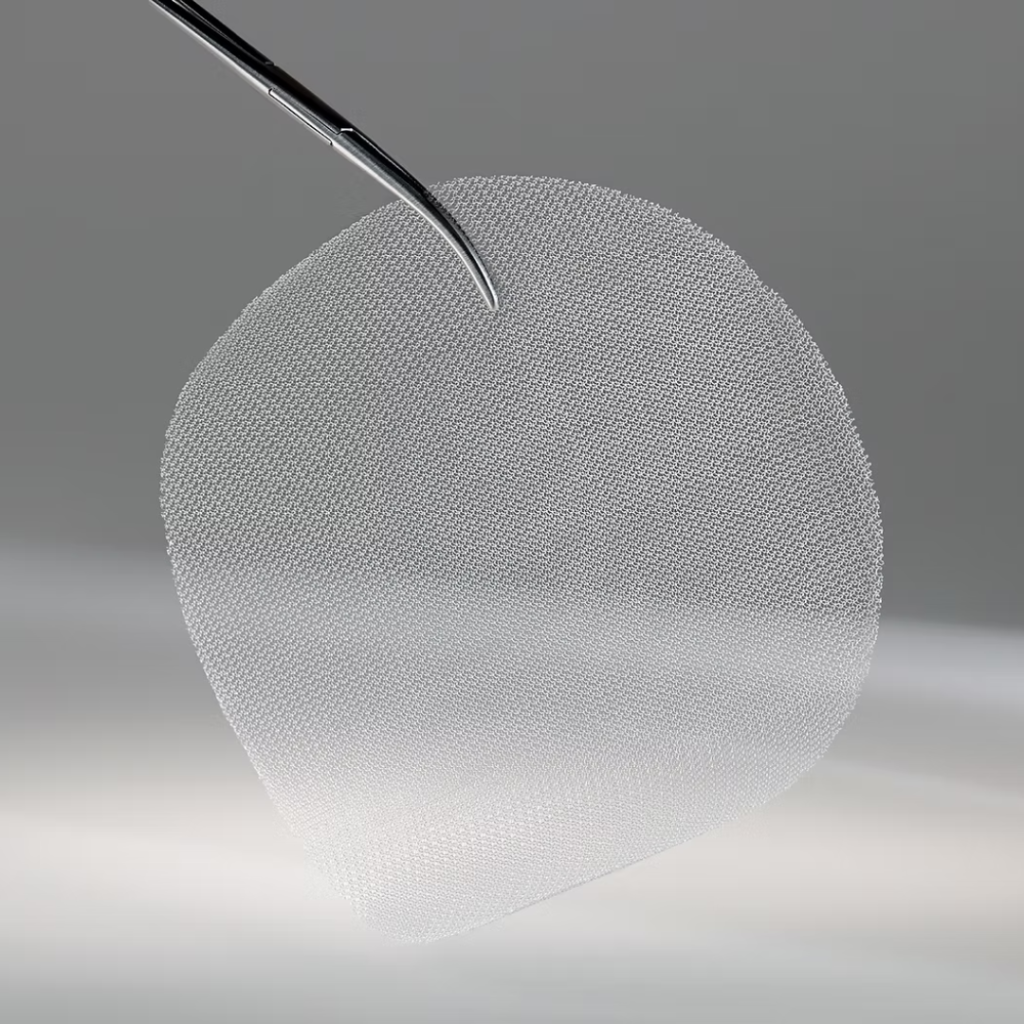
What Is the Study Device?
The device used in the study is a resorbable mesh made from P4HB, a biologically derived polymer. This device is used to provide temporary support and stability to the smooth silicone breast implant during revision surgery for capsular contracture, with the goal of reducing recurrence.
About the Capsular Contracture Study
This study is being conducted at multiple sites across the U.S. to determine the safety and effectiveness of the study device in treating capsular contracture. Dr. Newall and his team are actively enrolling qualified candidates to participate.

Who Is a Candidate? Who Is a Candidate?
- Are female, age 22–66
- Have capsular contracture (Baker Grade III or IV)
- Desire a new implant with no more than a ±150 cc volume change
- Are able to comply with the required follow-up visits for 24 months

Who Is Not a Candidate?
- BMI below 18 or above 35
- Implant size greater than 800 cc
- History of breast cancer, chest radiation, or certain autoimmune conditions
- Recent nicotine use, active infections, or systemic illness
- Plans for additional elective breast surgery during the study period
Benefits for Study Participants
Patients are eligible for potential benefits, including:
- Surgery fees waived (excluding anesthesia and facility fees)
- Study device [provided at no cost]
- Compensation for follow-up visits when completed as requested
By joining this study, you not only receive access to an advanced treatment option for capsular contracture, but you also play an important role in helping shape the future of breast implant revision surgery.
Request a ConsultationPotential Risks Include:
- Breast asymmetry (when one breast has a different size, volume, position, or form from the other)
- Allergic reaction to materials used to manufacture the mesh, including kanamycin and tetracycline, which might lead to serious health issues
- Allergic reaction to Betadine (a solution used to clean the pocket) in patients with a hypersensitivity to Betadine
- Breast/nipple sensitivity change (a change in what or how much you feel on/in your breast or nipple)
- Capsular contracture (when the scar tissue around the implant constricts)
- Breast deformity (when the breast does not have a normal or typical shape)
- Erosion (breakdown of the outer layers of the skin)
- Erythema (abnormal redness of the breast)
- Explantation: Implant + Scaffold (when the implant and the remaining scaffold need to be surgically removed)
- Scaffold extrusion (when the scaffold becomes exposed, when the incision doesn’t close correctly)
- Hematoma (collection of blood in tissues, like a very bad bruise)
- Imaging artifact(s) (when something is seen in a radiology picture (like an X-ray) that might make the picture difficult to interpret)
- Impaired wound healing (when one or more issues lead to delayed wound healing)
- Implant rupture/silicone bleed (when the implant breaks open and/or silicone leaks out)
- Infection
- Inflammation (swelling)
- Implant malposition (the implant not being in the right place / out of place)
- Necrosis (dead or dying tissue)
- Pain
- Ptosis (drooping or sagging of the breast)
- Skin Rash / Red Breast Syndrome (when the skin is red and inflamed; abnormal redness of the breast that can look like a skin infection)
- Scaffold/implant malposition (the implant or the scaffold not being in the right place / out of place)
- Scaffold palpability (being able to feel the scaffold through the skin)
- Scaffold visibility (being able to see the scaffold through the skin)
- Seroma (a collection of fluid under the skin, usually near the incision)
- Soft tissue defect
- Wound dehiscence (when the surgical wound partially or completely opens)
- There may be other potential unknown risks associated with the surgical procedure or study device, such as the safety and effectiveness of device usage in proximity to existing or excised cancer, or the impact on breastfeeding.
- There is a potential unknown impact on the ability to detect breast implant rupture, breast cancer, or other breast diseases.
What Follow-Up is Required?
Participants will be followed closely for 24 months to ensure the best possible outcomes. Required visits include:
- Post-Operative Visit (approx. 90 days post-op) – In-person exam, photos, and review of healing progress
- 6-Month Phone Visit – Virtual check-in to document healing, side effects, or life changes
- 12- and 24-Month Follow-Ups – In-person exams, breast imaging, questionnaires, and documentation of results
- Additional follow-up may be requested if necessary for safety or regulatory purposes.

Schedule a Consultation
If you believe you may qualify for this study, we invite you to schedule a private consultation with Dr. Newall. He will review your case, explain the study in detail, and determine if you’re an eligible candidate.
Take the first step toward relief from capsular contracture and explore this innovative treatment option with Dr. Newall today.
Request a Consultation